- This topic is empty.
-
AuthorPosts
-
2025-05-15 at 5:48 pm #4216
5-Bromovaleryl Chloride (CAS Number: 4509-90-4) is an important intermediate compound in organic chemistry, especially in the realm of pharmaceuticals, agrochemicals, and advanced material synthesis. This halogenated acyl chloride combines the reactive features of both an acyl chloride and an alkyl bromide, offering unique reactivity that allows it to be used in a broad spectrum of chemical transformations. In this blog post, SACH, a high purity fine chemicals manufacturing factory, will share 5-bromovaleryl chloride as raw material for chemical synthesis, including its molecular structure, physicochemical properties, reactivity profile, synthetic applications, and safety considerations.
5-Bromovaleryl Chloride Molecular Structure and Physicochemical Properties
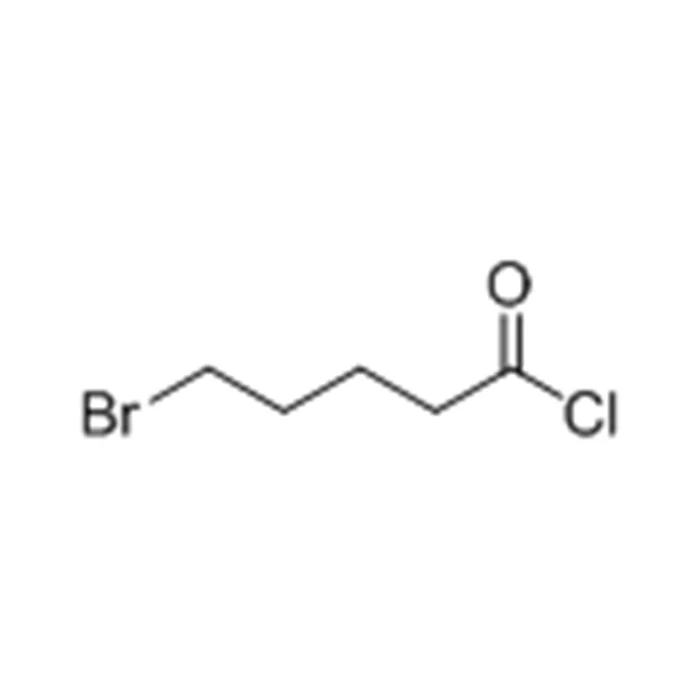
– IUPAC Name: 5-bromopentanoyl chloride
– CAS Number: 4509-90-4
– Molecular Formula: C5H8BrClO
– Molecular Weight: 199.473 g/mol
– Appearance: Colorless to pale yellow liquid
– Boiling Point: 214.1±0.0 °C at 760 mmHg
– Density: 1.5±0.1 g/cm3
– Solubility: Reacts with water; soluble in most organic solvents like dichloromethane, toluene, and chloroform
Structurally, 5-Bromovaleryl Chloride consists of a five-carbon linear chain terminated by an acyl chloride group on one end and a bromine atom on the fifth carbon. This dual functionality makes it a versatile bifunctional reagent for sequential reactions or tandem strategies in organic synthesis.
5-Bromovaleryl Chloride Reactivity Profile and Synthetic Utility
1. Acyl Chloride Functionality: The acyl chloride moiety is highly electrophilic and reactive toward nucleophilic substitution. It readily undergoes reactions with alcohols (to form esters), amines (to form amides), and water (to form carboxylic acids). This allows the introduction of the valeryl moiety into diverse molecular frameworks.
2. Bromoalkyl Functionality: The bromine atom on the terminal carbon provides a good leaving group for SN2-type nucleophilic substitutions and metal-halogen exchange reactions. This allows for further functionalization, including the formation of Grignard reagents, coupling reactions, or heteroatom substitution.
This bifunctional character makes 5-Bromovaleryl Chloride an excellent intermediate for multistep synthesis strategies, where sequential or orthogonal derivatization of the acyl and haloalkyl groups is desired.
Applications of 5-Bromovaleryl Chloride in Chemical Synthesis
1. Synthesis of β-Keto Amides and Esters
Due to its acyl chloride group, 5-Bromovaleryl Chloride serves as a precursor to β-keto amides and esters. When reacted with β-keto amines or alcohols, it forms derivatives that are structurally valuable in medicinal chemistry. These compounds often display bioactivity or serve as key intermediates in the synthesis of enzyme inhibitors and pharmaceuticals.
2. Introduction of Brominated Side Chains
The bromine at the terminal position can be substituted with various nucleophiles including thiols, amines, and alkoxides, allowing for the introduction of functional side chains. This property is useful in the design of molecular probes, surfactants, and other fine chemicals where tailored polarity and reactivity are needed.
3. Polymer Modification and Monomer Synthesis
5-Bromovaleryl Chloride can be employed in the synthesis of monomers or side-chain functionalization of existing polymers. The bromine atom enables atom transfer radical polymerization (ATRP) initiator synthesis, while the acyl chloride moiety allows for direct attachment to polymer backbones via ester or amide linkages.
4. Pharmaceutical Intermediate
In pharmaceutical chemistry, halogenated acyl chlorides like 5-Bromovaleryl Chloride are often used as intermediates in the synthesis of biologically active molecules. The presence of bromine can increase lipophilicity and membrane permeability, while the acyl chloride group introduces pharmacophoric carbonyl functionalities.
5. Peptide and Protein Labeling
Given its reactivity, 5-Bromovaleryl Chloride has potential applications in the selective modification of proteins and peptides through acylation. The bromine group enables site-specific labeling, bioconjugation, or tethering of reporter molecules in biochemical assays.

Synthetic Strategies Involving 5-Bromovaleryl Chloride
Several synthetic strategies benefit from the dual-reactive nature of 5-Bromovaleryl Chloride:
– Sequential Nucleophilic Substitution and Amidation: Starting with nucleophilic substitution on the bromide (e.g., azide displacement to generate an azido compound), followed by amidation of the acyl chloride, one can generate multifunctional intermediates.
– Grignard Reagent Formation: Treatment with magnesium yields a Grignard reagent that can be reacted with electrophiles, enabling carbon–carbon bond formation.
– Click Chemistry Precursor: The terminal bromo group can be converted into an azide, enabling Huisgen 1,3-dipolar cycloaddition with alkynes in click chemistry applications.
Handling and Safety Considerations of 5-Bromovaleryl Chloride
5-Bromovaleryl Chloride is a moisture-sensitive and corrosive compound. It reacts exothermically with water, alcohols, and bases, releasing HCl gas. Hence, strict safety precautions must be observed:
– Protective Equipment: Use of gloves, goggles, and a fume hood is essential.
– Storage: Store under inert gas (e.g., nitrogen) in a tightly sealed container, away from moisture and bases.
– Disposal: Residues and waste must be neutralized and disposed of in accordance with local environmental regulations.
Conclusion
5-Bromovaleryl Chloride (CAS 4509-90-4) is a highly versatile synthetic building block offering the dual functionality of a reactive acyl chloride and a terminal bromine. Its bifunctional nature allows for wide-ranging applications in pharmaceutical synthesis, polymer science, and functional material development. Due to its reactivity and synthetic utility, it remains a staple intermediate in the toolkit of synthetic organic chemists.
-
AuthorPosts
- You must be logged in to reply to this topic.
